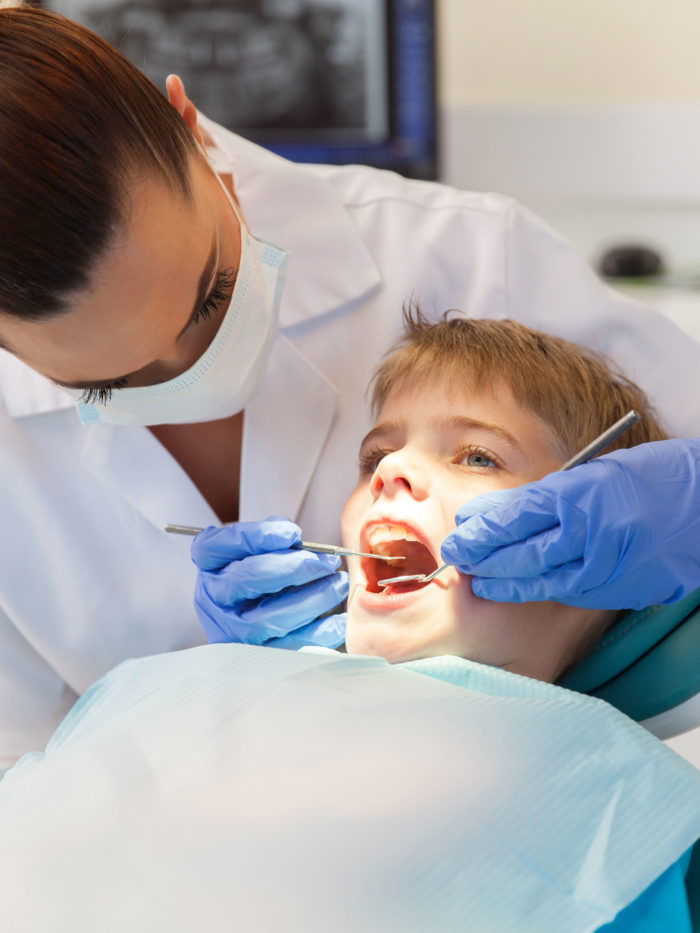
Years ago, a mysterious outbreak of oral infections was traced to a clinic in Anaheim alarmed parents and dominated the local news.
CHOC telehealth visits continue at a rapid pace
Virtual visits with a CHOC provider via a smart phone, tablet or computer are here to stay and expected to continue growing at a rapid pace.
Virtual pediatric lecture series: COVID-19 in children
CHOC virtual pediatric lecture series continues with a session on COVID-19 in children with Dr. Antonio Arrieta and Dr. Jasjit Singh.
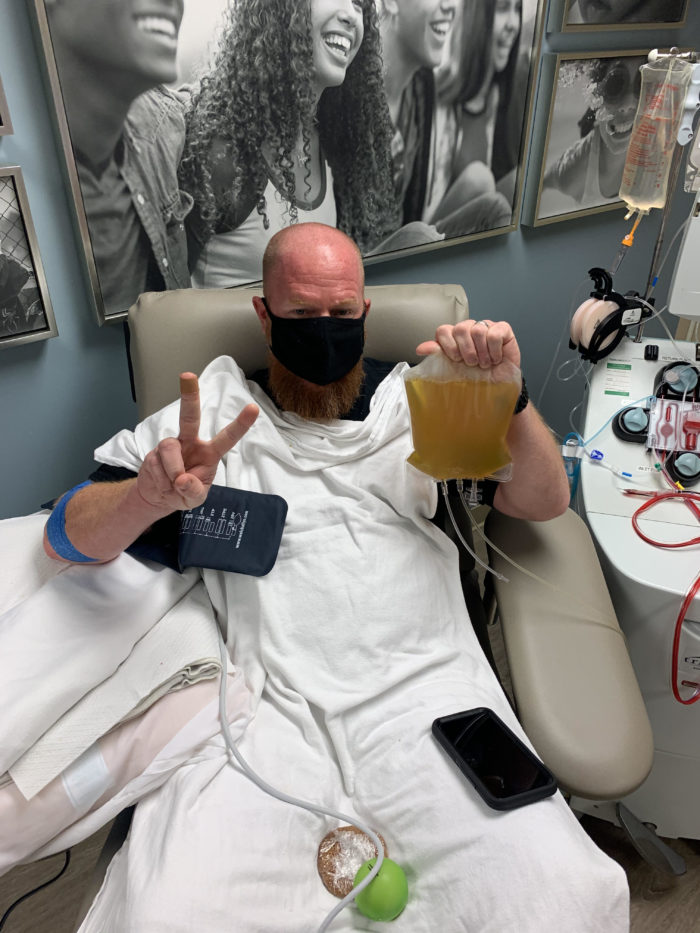
How COVID-19 survivors can support others through plasma donation
It’s possible that the plasma of recovered COVID-19 patients may have antibodies that could help in the treatment of others with COVID-19.
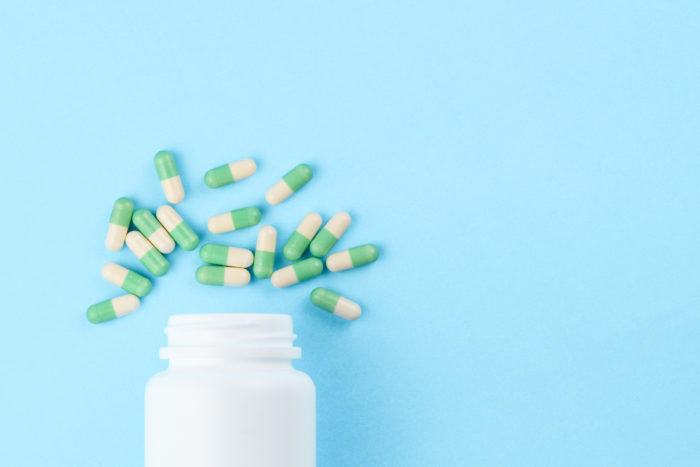
Leprosy antibiotic is safe treatment for M. abscessus infections, CHOC infectious disease team finds
An oral antibiotic used to treat leprosy is safe and well-tolerated in children with challenging-to-treat mycobacterium abscessus infections.
2015 CHOC – UC Irvine Child Health Research Awards
We are pleased to announce that we just completed another round of the CHOC – UC Irvine Child Health Research Awards, our annual call for proposals that enhance research collaborations between CHOC and UC Irvine and further the Mission, Vision and strategic aims of the CHOC-UCI Child Health Research Strategic Plan. Intended to support research […]