Training the next generation of talented pediatric research coordinators is an essential part of CHOC Research’s mission to support top-quality research that improves children’s lives and well-being. Chief Scientific Officer Dr. Terence Sanger says.
$2.8-million gift to fund device to tame movement disorder triggers in the brain
CHOC is one of the top nine centers in the country doing cutting-edge cerebellum research.
Planning the future together: CHOC Research Institute welcomes Rady at 2025 strategy retreat
The annual conference signals a new era under the Rady Children’s Health umbrella.
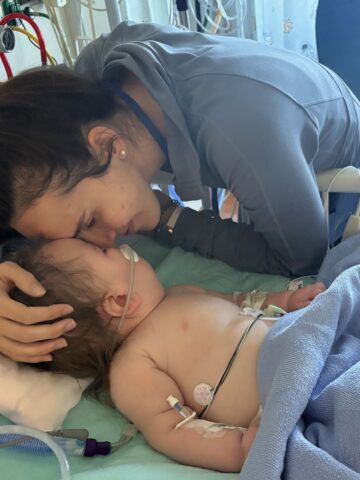
Parents of 1-year-old with exceedingly rare and life-threatening mitochondrial disease entrust CHOC above anywhere else
With time not on their side, CHOC care team pulls out all stops to save life of Emery Kline.
Experts address the most pressing challenges at Pediatric & Lifespan Data Science Conference
Panel discussions also were held on precision medicine, fairness in AI, precision management of psychiatric conditions, and other issues.
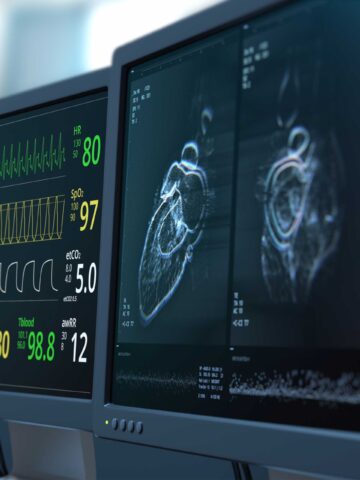
CHOC-led study explores cardiac MRI for borderline left ventricle surgery
The new study is an example of how CHOC and UCLA Health are transforming the way cardiac care is being delivered in Southern California.